The CDC announced Thursday that it has closed its baby formula investigation due to contamination concerns, with no additional cases identified as part of the investigation, according to the FDA’s website.
What You Need To Know
- The CDC announced that it has closed its baby formula investigation due to contamination concerns
- No additional cases have been identified as part of the investigation
- Although the investigation has come to an end, baby formula shortage problems continue to remain, and the recall is still in effect
There has been a short supply of baby formula nationwide for months, due to pandemic-related supply chain issues. That shortage then reached crisis levels after four children became sick, and two died, after suspected bacterial contamination of formula which originated from Abbot Nutrition’s formula plant in Sturgis, Michigan. The FDA’s Coordinated Outbreak Response and Evaluation (CORE) Network, along with the CDC and state and local partners investigated the issue.
According to the FDA, CORE is no longer investigating the incident, but the FDA established an Incident Management Group (IMG) on April 1, 2022, to continue to work on supply chain and food safety issues.
However, although the investigation has come to an end, baby formula shortage problems continue to remain around the country, and the recall is still in effect.
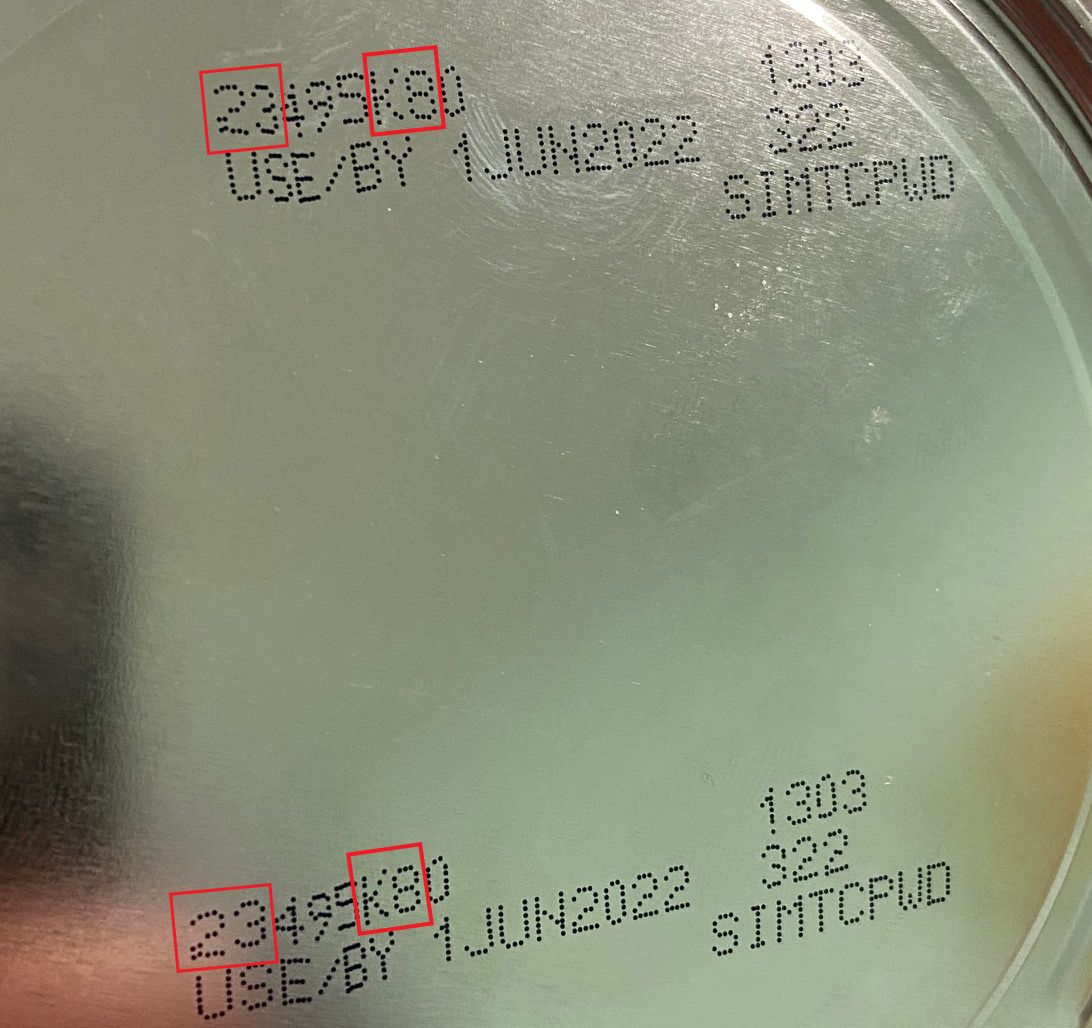
The FDA is advising consumers not to use recalled Similac, Alimentum, or EleCare powdered infant formulas. Recalled products can be identified by the 7 to 9 digit code and expiration date on the bottom of the package (see image below). Products are included in the recall if they have all three items below:
- the first two digits of the code are 22 through 37 and
- the code on the container contains K8, SH, or Z2, and
- the expiration date is 4-1-2022 (APR 2022) or later.
_(2))
(FDA.gov)
)
(FDA.gov)

(FDA.gov)
In addition to products described above, Abbott Nutrition has recalled Similac PM 60/40 with a lot code 27032K80 (can) / 27032K800 (case). At this time, Similac PM 60/40 with lot code 27032K80 (can) / 27032K800 (case) is the only type and lot of this specialty formula being recalled. Additional recall information for the initial recall is available on the FDA website. Parents can also enter their product lot code on the company’s website to check if it is part of the recall.
The recalls do not include liquid formula products.
Parents and caregivers should never dilute infant formula and should not make or feed homemade infant formula to infants. Consumers should also avoid purchasing imported formula through online sales, as it has the potential to be counterfeit.
If your regular formula is not available, contact your child’s healthcare provider for recommendations on changing feeding practices.
If you get infant formula through WIC, do not throw the formula out. Instead, you should take it to the store for a refund and exchange or call the company at 1-800-986-8540 to help you. WIC recipients should be able to obtain a different brand of similar formula. Call your local WIC clinic for more guidance. Also see:
More information on Cronobacter and infant formula is available on CDC’s website.