COLUMBUS, Ohio — Johnson & Johnson’s vaccine is expected to boost the sluggish pace of inoculations in the U.S., and states are now finalizing their plans to disseminate a drug that offers significant protection, but is inferior to already authorized mRNA vaccines.
To date, those in priority groups for COVID-19 immunization haven’t had much of a choice between the Pfizer-BioNTech and Moderna shots, and officials have decided where to ship which vaccine based on logistical considerations more than anything else.
That could change when Johnson & Johnson’s 66% effective vaccine is rolled out alongside two vaccines that are 90%-plus effective, said Jonathan Karn, Case Western University School of Medicine’s chair of microbiology and molecular biology.
The FDA plans to meet Friday to decide if the vaccine receives emergency use authorization. If it does, it could be distributed as soon as Saturday, according to health officials. Its arrival will bring about new strategic considerations for public health officials locally and nationwide.
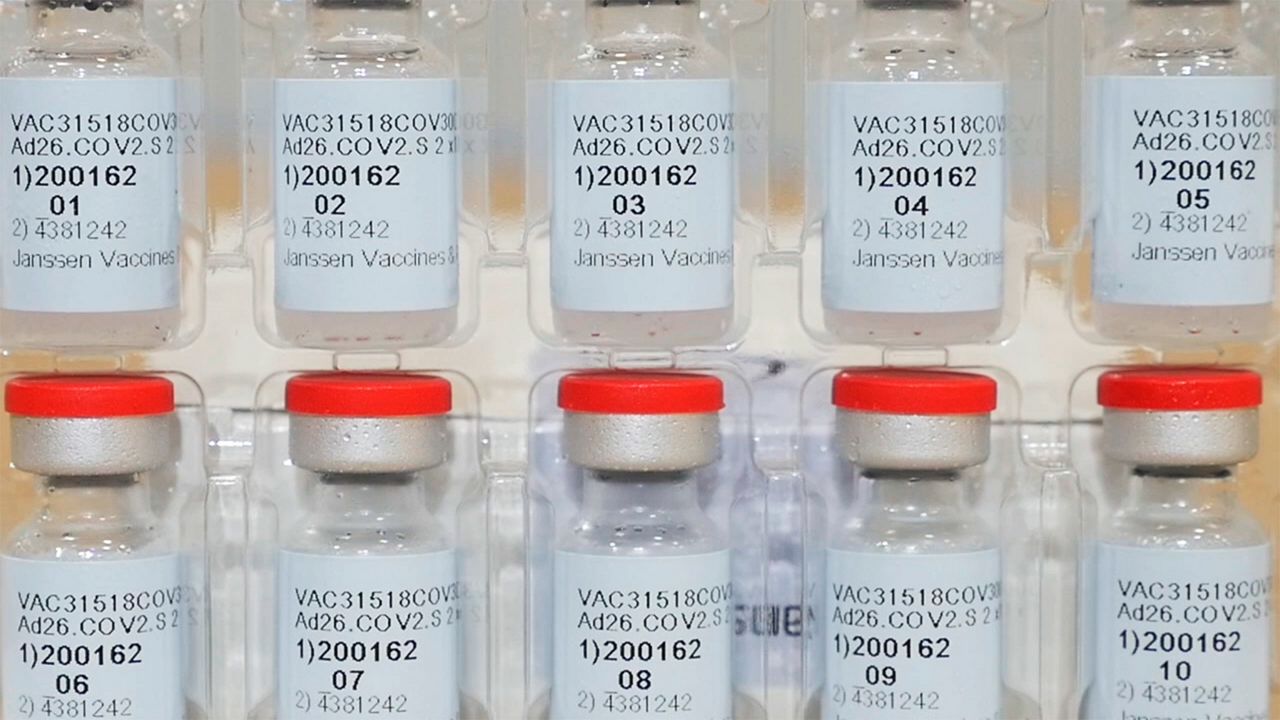
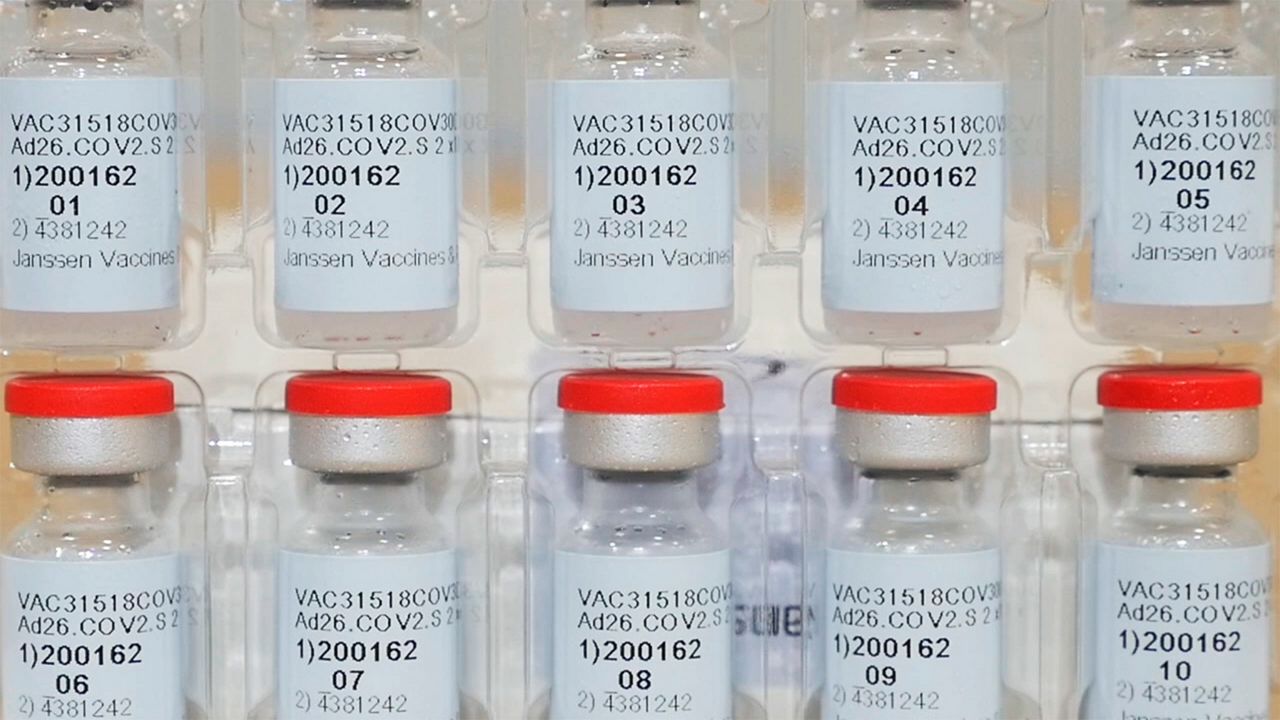
This Dec. 2, 2020, photo provided by Johnson & Johnson shows vials of the Janssen COVID-19 vaccine in the United States. (Johnson & Johnson via AP)
Karn’s suggestion: Give seniors the “magic sauce” — the Pfizer and Moderna vaccines — and use the still efficacious, but far less effective Johnson & Johnson vaccine to hit groups like essential workers, who are at high risk of contracting and transmitting the virus, but not so high risk of serious illness or death.
“Reserve the mRNA vaccines for the older populations who are most at risk,” he suggested. “And do broader vaccine campaigns with this other vaccine, which may be a little less efficacious.”
Johnson & Johnson will be authorized without a booster, though the company is evaluating a two-dose regimen. With a single-shot vaccine, states could make a dent in the spread of the virus, jabbing residents from aging from their teens to their 40s in essential industries getting hit hard by the pandemic, Karn said.
“Strategically, for the state or the country marshaling their resources, they may elect to do it in this kind of stratified way,” he said.
Karn recommends getting the Johnson & Johnson vaccine if it’s available to you rather than waiting for Pfizer or Moderna.
If not for the remarkable effectiveness of the Pfizer and Moderna vaccines, the medical community would be rejoicing at a 66% effective COVID-19 vaccine, he said.
However, Karn said there is one other potential inferiority of the Johnson & Johnson vaccine if COVID-19 sticks with us into 2022 and beyond: The virus could become an endemic like the flu, which he is increasingly confident will be the case as he watches the emergence of variants around the globe.
“There are these variants that are arising, and they’re rising all over the globe. And so just like flu, it’s reasonable to predict that there’ll be new variants that show up, and they may be partially resistant or even fully resistant to this existing vaccine,’ Karn said.
Across the globe, it’s likely booster shots of the vaccine will be administered every year or every two years as the vaccine’s protection wanes, he said.
“If you think then about, well, what are the best vaccine platforms to do that? Then there is some superiority for the messenger RNA vaccines over the J&J type of vaccines,” he said.
If people need boosters each year, the Pfizer and Moderna vaccines are the gold standard, he said.
To respond to viral mutations, the vaccines can be tweaked quickly, as evidenced by Moderna’s announcement Wednesday the company has already developed a new version of its vaccine for the variant first discovered in South Africa.
“The advantage of the RNA vaccines is that they’re extremely flexible, so just as you can program for the circulating COVID strains, you can make a small variant, and just like flu come up with a cocktail for regular revaccination,” he said.
The Pfizer and Moderna vaccines insert messenger RNA to the muscle cells where it produces the viral spike protein, causing the immune system to start making antibodies to the foreign protein. With this type of vaccine platform, experts anticipate recurring annual or biennial revaccination to be just as efficacious as the first shot, Karn said.
But Johnson & Johnson’s vaccine is an adenovirus vaccine, which is a very different technology with some drawbacks.
“When you get vaccinated with these viral vectors, you make an immune response to the carrier virus as well as to the COVID protein that you’re trying to vaccinate with, and the consequence of that is that you can’t use them again and again and again. You get a few reuses, but then their efficacy tends to fade,” he said.
While the Johnson & Johnson vaccine is expected to have what he calls “second-generation reuse issues,” experts do not expect it to be a problem to get that vaccine this year and then start an mRNA regimen for revaccination down the line.
And for now, there’s good news: “The line is holding.” Karn said that the vaccines have the upper hand on the variants at the moment, preventing severe disease, if not infection.
The concern is the pace of mutations that has been seen this winter.
“This is quite literally an evolving situation. The virus is evolving, and so the long-term worry is that somewhere around the globe, there will be a variant that emerges that is effective at escaping the vaccine,” Karn said.
The virus could also mutate to become more deadly. Other coronaviruses are neuroinvasive, and that type of mutation on a disease as contagious as COVID-19 could be devastating.
“That’s the sort of nightmare that keeps people like me up at night,” Karn said.