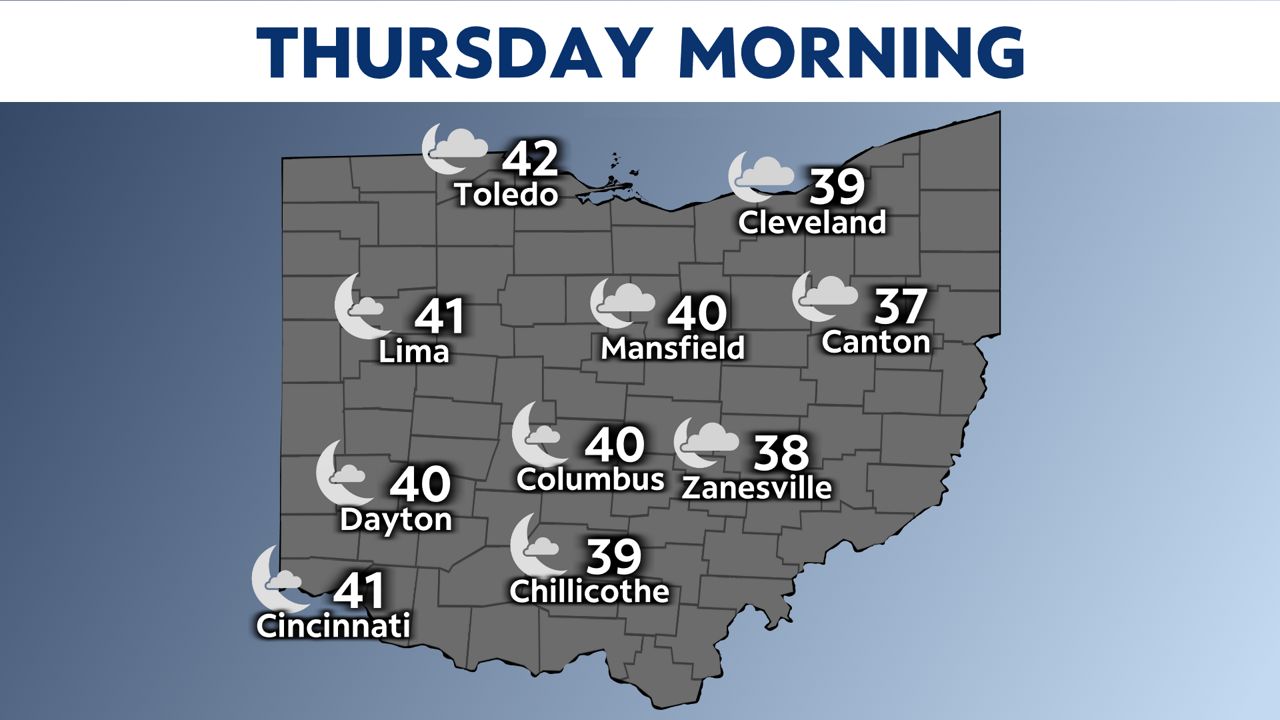
Canada authorized Pfizer-BioNTech’s COVID-19 vaccine for use in children aged 12 to 15 on Wednesday, officials announced in a statement.
The vaccine is the first to receive approval for use in the 12-15 age group in Canada. Pfizer-BioNTech received interim authorization from Health Canada to use its vaccine in people 16 years and older on Dec. 9, 2020.
“Today’s expansion of our authorization represents a significant step forward in helping the Canadian government broaden its vaccination program and begin to help protect adolescents before the start of the next school year,” Fabien Paquette, vaccines lead at Pfizer Canada, said in a statement.
“We are proud that we are playing a part of the efforts to help protect adolescent Canadians in this devastating pandemic,” he added.
Canada’s health department expanded the age group based on data from a Phase 3 clinical trial of 2,260 teens aged 12-15 in the United States. There is an ongoing study into the efficacy of the Pfizer vaccine in as young as six months old to 11 years of age.
The data showed there were no cases of COVID-19 among fully vaccinated adolescents compared with 18 among those given dummy shots.
Kids had side effects similar to young adults, the company said. The main side effects are pain, fever, chills and fatigue, particularly after the second dose. The study will continue to track participants for two years for more information about long-term protection and safety.
The U.S. Food and Drug administration is set to make a similar move in the coming weeks, according to a federal official and a person familiar with the process, setting up shots for many before the beginning of the next school year.
The announcement is set to come a month after the company found that its shot, which is already authorized for those age 16 and older in the United States, also provided protection for the younger group.
The federal official, speaking on the condition of anonymity to preview the FDA’s action, said the agency was expected to expand its emergency use authorization for Pfizer’s two-dose vaccine by early next week, and perhaps even sooner. The person familiar with the process, who spoke on condition of anonymity to discuss internal matters, confirmed the timeline and added that it is expected that the FDA will approve Pfizer’s use by even younger children sometime this fall.
Pfizer isn’t the only company seeking to lower the age limit for its vaccine. Results also are expected by the middle of this year from a U.S. study of Moderna’s vaccine in 12- to 17-year-olds.
The New York Times first reported on the expected timing for Pfizer’s authorization for younger age groups within the United States.
The Associated Press contributed to this report.